CORROSION TYPES And Prevention
Posted on June, 2017
In a previous post, we discussed the basics of corrosion -- from the fundamental chemical reaction to the types of environments in which corrosion can occur. As corrosion most often occurs in aqueous environments, we now explore the different types of degradation a metal can experience in such conditions:
Uniform Corrosion
Uniform corrosion is considered an even attack across the surface of a material and is the most common type of corrosion. It is also the most benign as the extent of the attack is relatively easily judged, and the resulting impact on material performance is fairly easily evaluated due to an ability to consistently reproduce and test the phenomenon. This type of corrosion typically occurs over relatively large areas of a material’s surface.
Pitting Corrosion
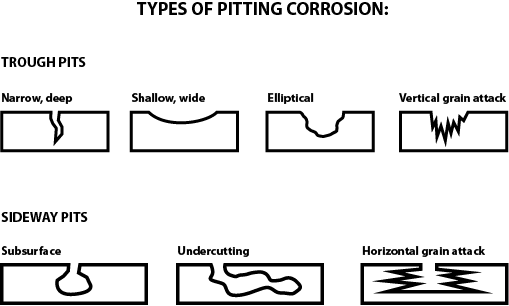
Pitting is one of the most destructive types of corrosion, as it can be hard to predict, detect and characterize. Pitting is a localized form of corrosion, in which either a local anodic point, or more commonly a cathodic point, forms a small corrosion cell with the surrounding normal surface. Once a pit has initiated, it grows into a “hole” or “cavity” that takes on one of a variety of different shapes. Pits typically penetrate from the surface downward in a vertical direction. Pitting corrosion can be caused by a local break or damage to the protective oxide film or a protective coating; it can also be caused by non-uniformities in the metal structure itself. Pitting is dangerous because it can lead to failure of the structure with a relatively low overall loss of metal.
Crevice Corrosion
Crevice corrosion is also a localized form of corrosion and usually results from a stagnant microenvironment in which there is a difference in the concentration of ions between two areas of a metal. Crevice corrosion occurs in shielded areas such as those under washers, bolt heads, gaskets, etc. where oxygen is restricted. These smaller areas allow for a corrosive agent to enter but do not allow enough circulation within, depleting the oxygen content, which prevents re-passivation. As a stagnant solution builds, pH shifts away from neutral. This growing imbalance between the crevice (microenvironment) and the external surface (bulk environment) contributes to higher rates of corrosion. Crevice corrosion can often occur at lower temperatures than pitting. Proper joint design helps to minimize crevice corrosion.
Intergranular Corrosion
An examination of the microstructure of a metal reveals the grains that form during solidification of the alloy, as well as the grain boundaries between them. Intergranular corrosion can be caused by impurities present at these grain boundaries or by the depletion or enrichment of an alloying element at the grain boundaries. Intergranular corrosion occurs along or adjacent to these grains, seriously affecting the mechanical properties of the metal while the bulk of the metal remain intact.
An example of intergranular corrosion is carbide precipitation, a chemical reaction that can occur when a metal is subjected to very high temperatures (e.g., 800°F - 1650°F) and/or localized hot work such as welding. In stainless steels, during these reactions, carbon “consumes” the chromium, forming carbides and causing the level of chromium remaining in the alloy to drop below the 11% needed to sustain the spontaneously-forming passive oxide layer. 304L and 316L are enhanced chemistries of 304 and 316 stainless that contain lower levels of carbon, and would provide the best corrosion resistance to carbide precipitation.
Stress Corrosion Cracking (SCC)
Stress corrosion cracking (SCC) is a result of the combination of tensile stress and a corrosive environment, often at elevated temperatures. Stress corrosion may result from external stress such as actual tensile loads on the metal or expansion/contraction due to rapid temperature changes. It may also result from residual stress imparted during the manufacturing process such as from cold forming, welding, machining, grinding, etc. In stress corrosion, the majority of the surface usually remains intact; however, fine cracks appear in the microstructure, making the corrosion hard to detect. The cracks typically have a brittle appearance and form and spread in a direction perpendicular to the location of the stress. Selecting proper materials for a given environment (including temperature and management of external loads) can mitigate the potential for catastrophic failure due to SCC.
Galvanic Corrosion
Galvanic corrosion is the degradation of one metal near a joint or juncture that occurs when two electrochemically dissimilar metals are in electrical contact in an electrolytic environment; for example, when copper is in contact with steel in a saltwater environment. However, even when these three conditions are satisfied, there are many other factors that affect the potential for, and the amount of, corrosion, such as temperature and surface finish of the metals. Large engineered systems employing many types of metal in their construction, including various fastener types and materials, are susceptible to galvanic corrosion if care is not exercised during the design phase. Choosing metals that are as close together as practicable on the galvanic series helps reduce the risk of galvanic corrosion.
Conclusion
In aqueous environments, metals may be exposed to not only uniform corrosion, but also to various types of local corrosion including pitting, crevice, intergranular, stress, and galvanic corrosion. In areas where corrosion is a concern, stainless steel products offer value and protection against these threats. Stainless’ favorable chemical composition makes it resistant to many common corrosives while remaining significantly more affordable than specialty alloys such as titanium and Inconel® alloys.
Stainless steel is a highly alloyed, low-carbon steel with a high (at least 11%) chromium content. When exposed to an oxygenated environment, the chromium reacts to form a passive oxide layer on the metal’s surface, slowing further oxidation and providing a self-healing quality, which helps resist uniform and local corrosion. Nickel helps to stabilize the microstructure, increasing SCC resistance. Manganese, in moderate quantities and in association with nickel, will perform many functions attributable to nickel and helps prevent pitting. The addition of molybdenum (the additional element in Type 316 SS that increases its performance with respect to Type 304 SS), helps increase resistance to pitting and crevice corrosion. Reduced levels of carbon, such as those found in 304L and 316L will help prevent intergranular corrosion. Lastly, nitrogen, although not a major element of stainless steel’s composition, increases pitting resistance. Choosing stainless steel can help greatly reduce the risk of corrosion and yield long-term savings by avoiding the costs associated with reinstallation of inferior products.

 Print This Page
Print This Page Email This Page
Email This Page